8 (4) A, C 0.1 gm of organic compound was analysed by Kjeldahl's method. In analysis produced NH, absorbed in 30 ml N/5 H,SO. The remaining acid required 20 ml N/10 NaOH
Por um escritor misterioso
Descrição
Click here:point_up_2:to get an answer to your question :writing_hand:84 a c01 gm of organic compound was analysed bykjeldahls method in analysis produced nhabsorbed
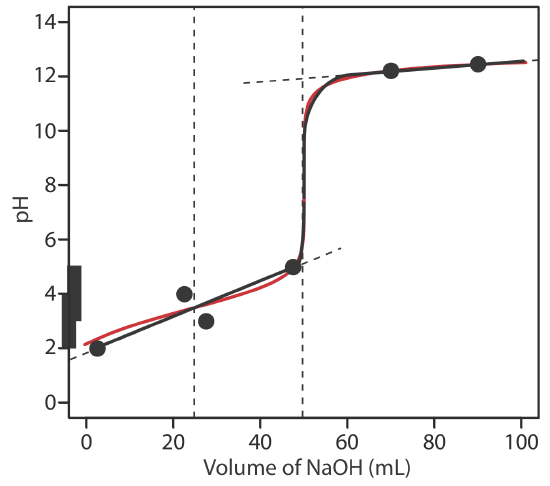
9.2: Acid–Base Titrations - Chemistry LibreTexts
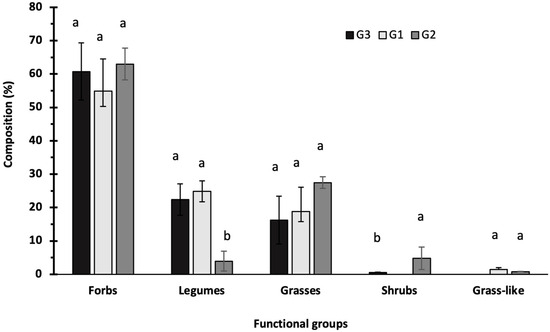
Dairy, Free Full-Text

0.35 g of an organic substance was Kjeldahlised and the ammonia obtained was passed into 100ml of M/10H_2SO_4 the excess acid required 154ml of M/10NaOH neutralisation calculate the % of nitrogen in

During nitrogen estimation present in an organic compound by Kje

02 - Practical Organic-Chem, PDF, Cyanide

DPP (1 TO) Physical Chemistry

NR. Ammonia obtained from 0.4 g of an organic substance by Kjeldahl's method was absorbed in 30 ml. of 0-25 MH SO. The excess of the acid was neutralized by the addition

NR. Ammonia obtained from 0.4 g of an organic substance by Kjeldahl's method was absorbed in 30 ml. of 0-25 MH SO. The excess of the acid was neutralized by the addition

0.4 g of an organic compound on Kjeldahi's enough ammonia to just neutraliz calculate the percentage of nitrogen in the c compound on Kjeldahl's analysis gave to just neutralize 20 mL of
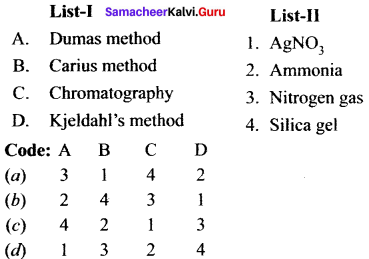
Samacheer Kalvi 11th Chemistry Solutions Chapter 11 Fundamentals of Organic Chemistry – Samacheer Kalvi

Co-operative Ecological Research Project (CERP), phase II: China - (mission). Project findings and recommendations

Study Notes on Analytical Chemistry, EB 3247 - Analytical Chemistry - Nilai

PDF) 242 Water, Nutrient, and Acid Requirements for Crops Grown Hydroponically in a Celss