ASAS-HI improvement ≥30%, ASDAS LDA status and ASAS40 response
Por um escritor misterioso
Descrição

Long-Term Safety and Efficacy of Ixekizumab in Patients With Axial Spondyloarthritis: 3-year Data From the COAST Program

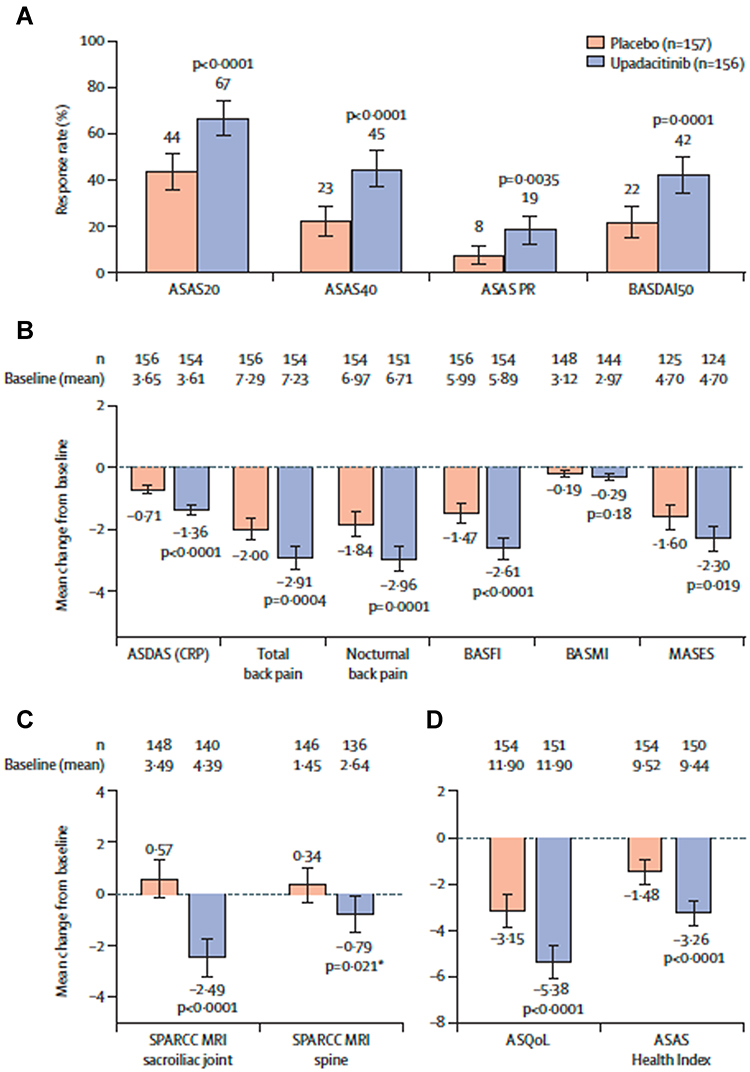
Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial

Full article: Effectiveness of bDMARDs in ankylosing spondylitis patients by biologic use: experience from the CorEvitas PsA/SpA Registry

Long-Term Safety and Efficacy of Ixekizumab in Patients With Axial Spondyloarthritis: 3-year Data From the COAST Program
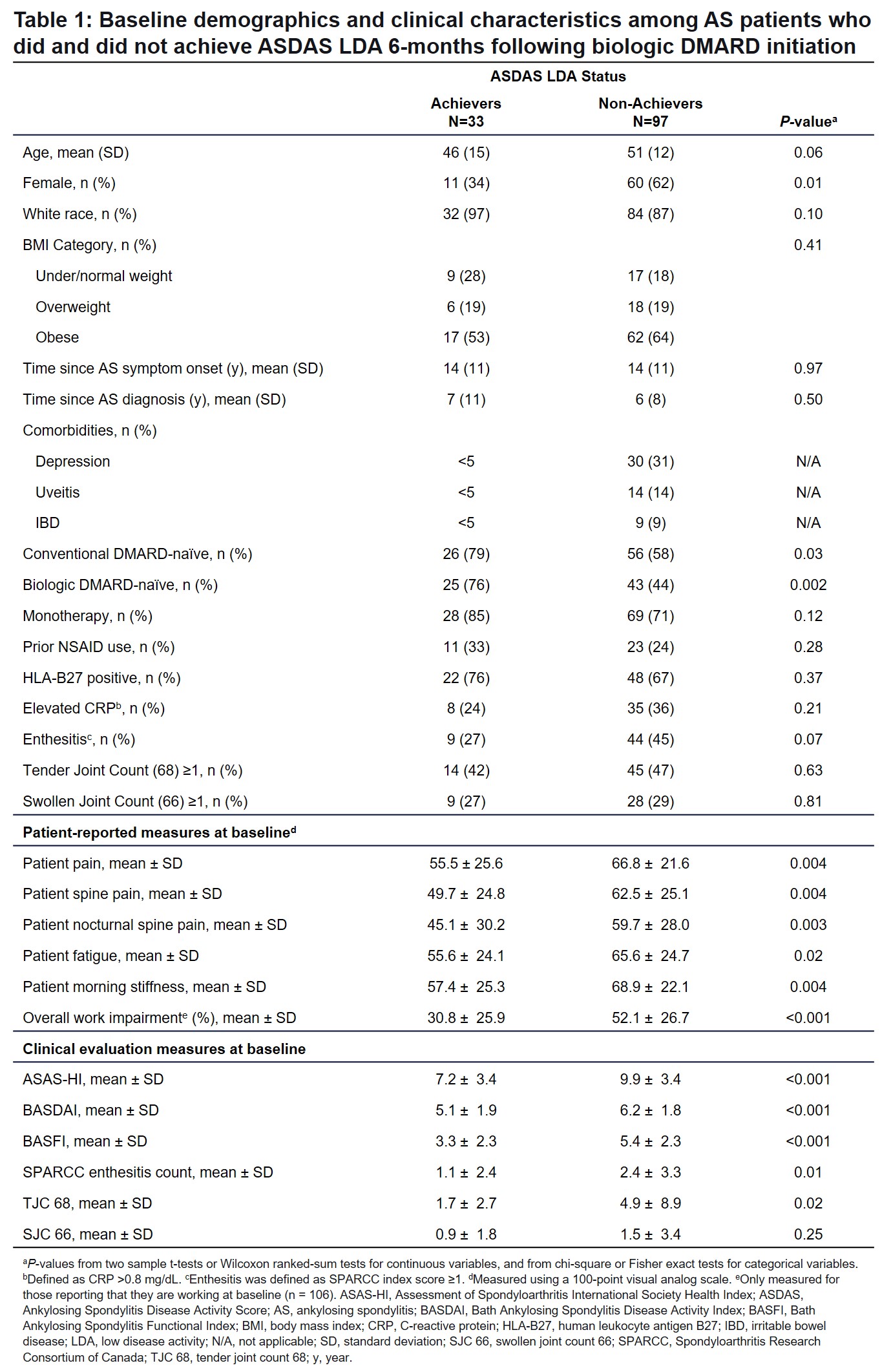
Impact of Achieving ASDAS LDA on Disease Activity and Patient-Reported Outcome Measures Among Patients with Ankylosing Spondylitis Treated with Biologic DMARDs - ACR Meeting Abstracts

Assessment of SpondyloArthritis international Society criteria for 20%

Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: results of the open-label, pragmatic, cluster-randomised TICOSPA trial
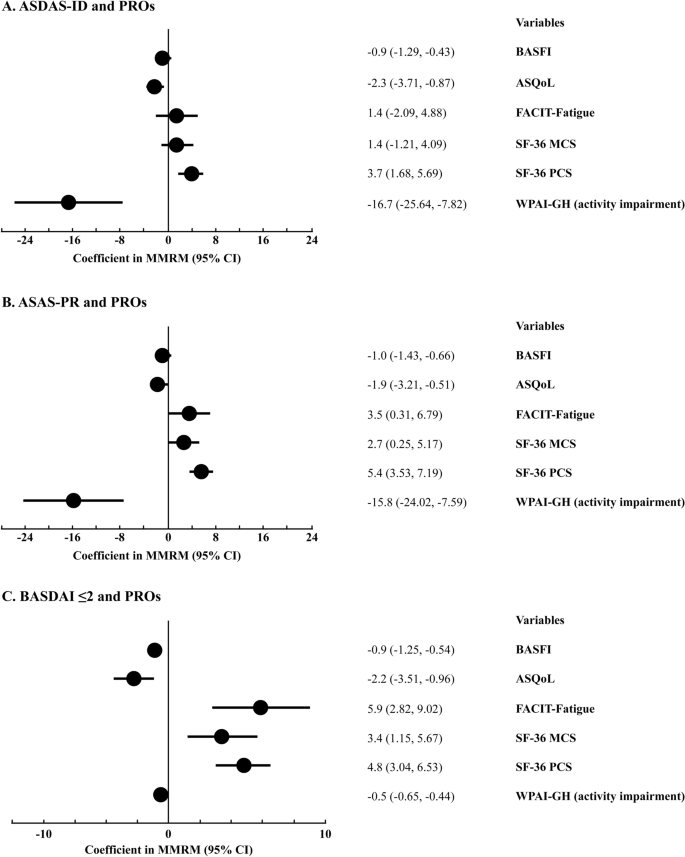
Achievement of Remission Endpoints with Secukinumab Over 3 Years in Active Ankylosing Spondylitis: Pooled Analysis of Two Phase 3 Studies
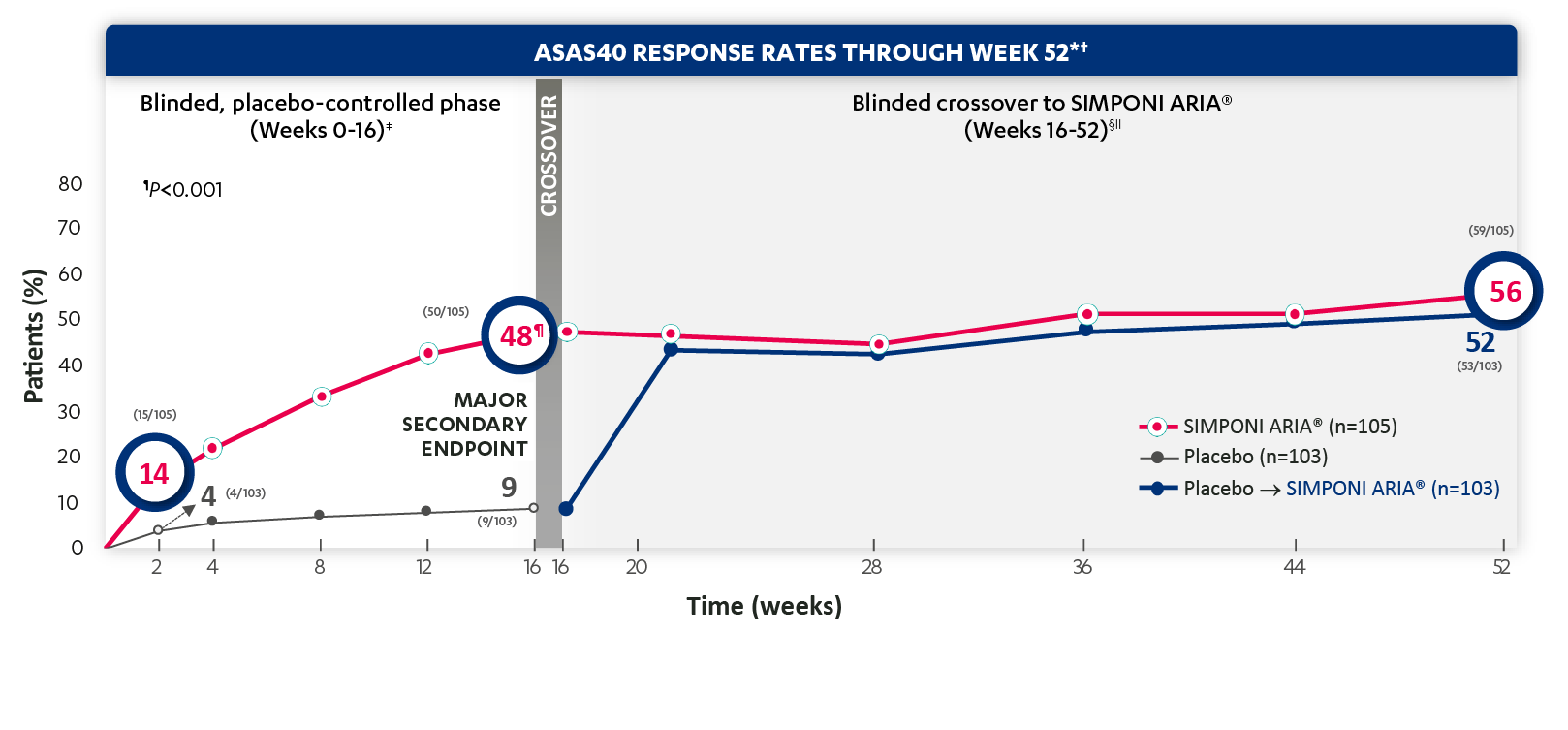
SIMPONI ARIA® Ankylosing Spondylitis: ASAS Response Rates

AS Criteria: Diagnosis (ASAS), Disease Activity (ASDAS), Radiographic Progression (mSASSS) - Arthritis Rheumatism
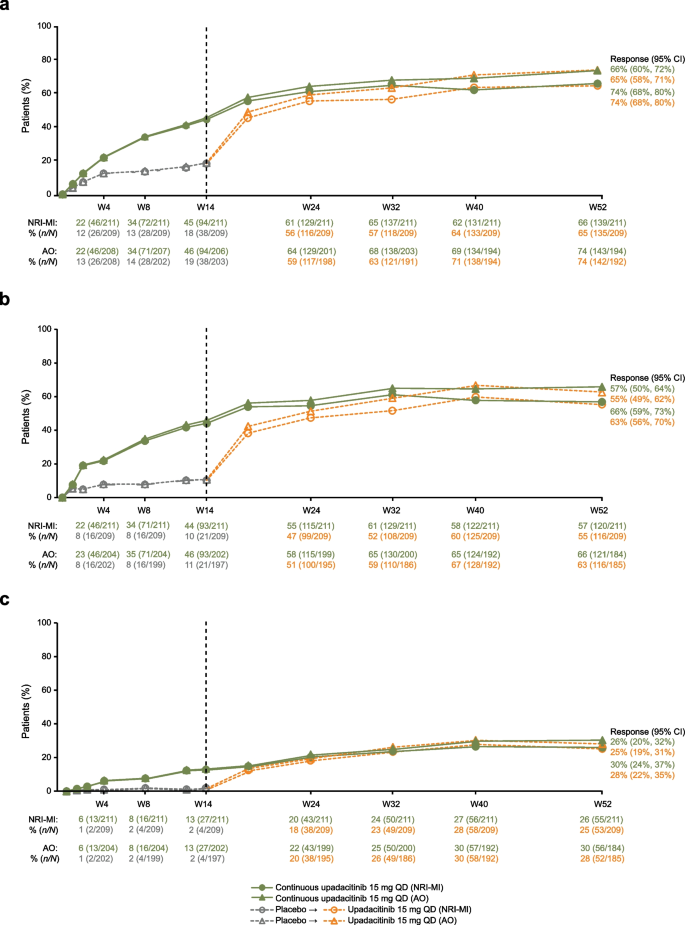
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy
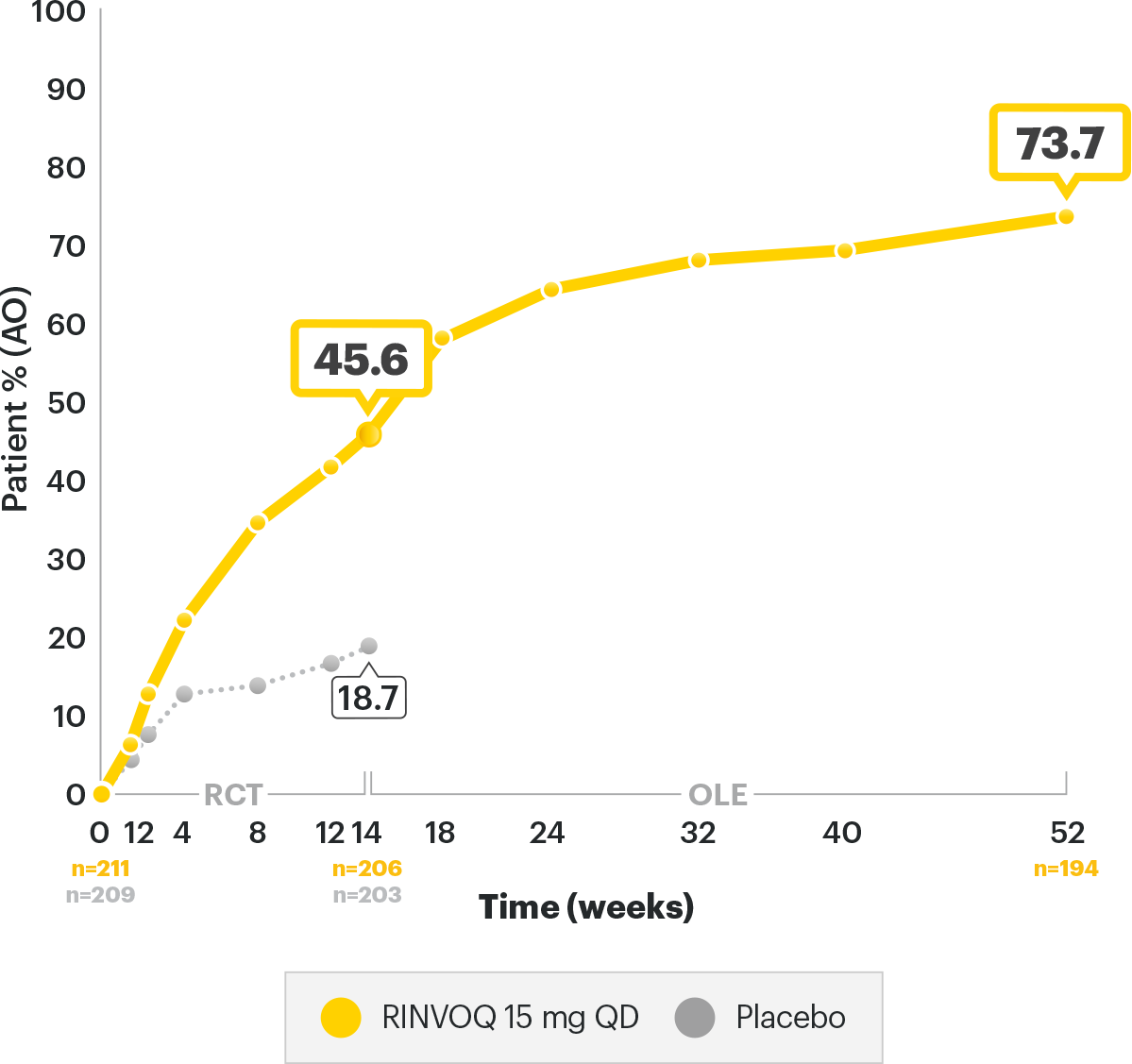
Disease Control Data, Ankylosing Spondylitis
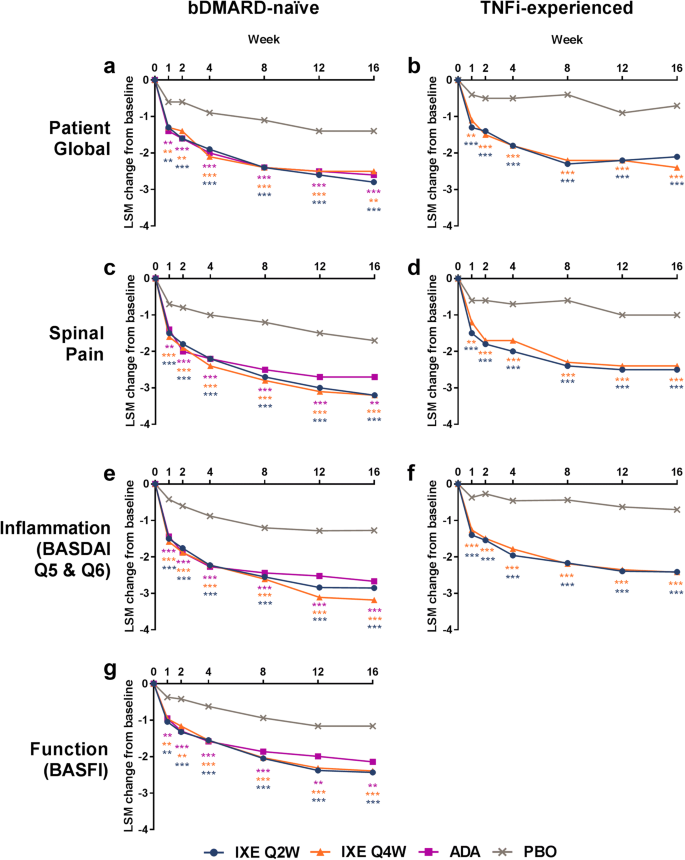
Translating Improvements with Ixekizumab in Clinical Trial Outcomes into Clinical Practice: ASAS40, Pain, Fatigue, and Sleep in Ankylosing Spondylitis
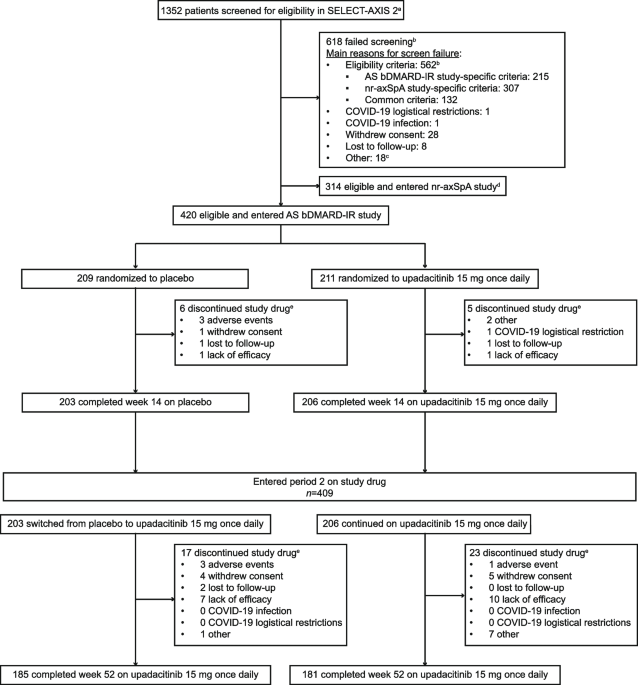
Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study, Arthritis Research & Therapy
Management of axial spondyloarthritis